half life formula for first order reaction
For a first-order reaction the half. 2 k t 1 2.
What is the expression for Half-Life of a First Order ReactionHere I derive it from the integrated rate lawThe answer is t ln 2 kAsk me questions.

. Determining a half life. Answer 1 of 4. Substituting the values in the.
Ln 12A0A0. Graphical relations and half lives. Equations for Half Lives.
Get the free Half Life Calculator first order reaction widget for your website blog Wordpress Blogger or iGoogle. 453 t 1 2 0693 k. The half-life of a reaction is defined as the time it takes for one half of a reactant to disappear.
The rate law for this first order rate equation is where A is the concentration of radium-223 at time. The half-life of a second-order reaction is given by the formula 1kR 0. For the first order reaction you can plug the definition of the half life into the concentration-time reaction to obtain a neat relationship.
Half Life of First Order Reactions First-Order Reactions We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate. 2 0693 into the equation results in the expression for the half-life of a first-order reaction. The half-life of a first-order reaction does not depend upon the concentration of the reactant.
Thus for a first-order reaction each successive half-life is the same length of time as shown in Figure 45. On May 18 Biden invoked the Defense Production Act to speed up the. First-order reactions include those in radioactive decay.
Converting a half life to a rate constant. We know that- Integrated rate law- ln A lnAo - kt. Find more Chemistry widgets in WolframAlpha.
A first-order reactions half-life is a constant that is correlated with its rate constant. D A d t k A If the volume is V t then A n V t. Evaluate the half-life of a first-order chemical reaction for which the rate constant is given as eq22 times 10-4 rm s-1.
It is a constant and related to the rate constant for the reaction. T 12 0693k. The half-life of a zero-order reaction can be calculated by rearranging the equation.
The half-life is given the symbol t12 to denote that it is the time at which the. Half life is a particular phenomenon that takes place every day in various chemical reactions as well as nuclear reactions. The half-life of a first-order reaction is given as t 12 0693k.
The half-life of a reaction is the time required for a reactant to reach one-half its initial concentration or pressure. For a first-order reaction the half-life of a reactant is independent of its initial concentration. T 12 R 0 2k.
A first order reaction A -- B. Where k is the rate constant t is time A is molar concentration of reactant A at some time t during the. For first order reactions assuming the reaction A B C.
You will have to substitute A in the differential equation. T ½ A o 2k For a. The first-order reaction half-life equation is given by k 2303 t l o g R 0 R From the definition of the half-life of a first-order reaction at t t12 and R R 02.
Half-life refers to the amount of time it takes for half of a particular. For a zero order reaction A products rate k. Derivation of Half-Life Formula for First Order Reaction.

Half Life Of Zero Th 0th Order Reaction Derivation Youtube

Determine The Half Life Of A First Order Reaction Youtube

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200s 1 B 2 Mi N 1 C 4years 1
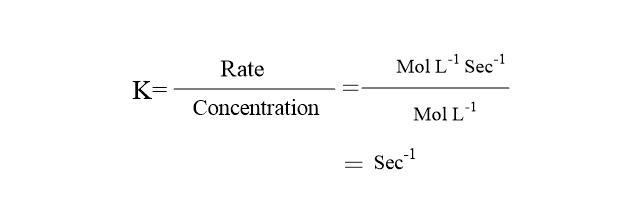
First Order Reaction Definition Example Half Life Period Chemist Notes
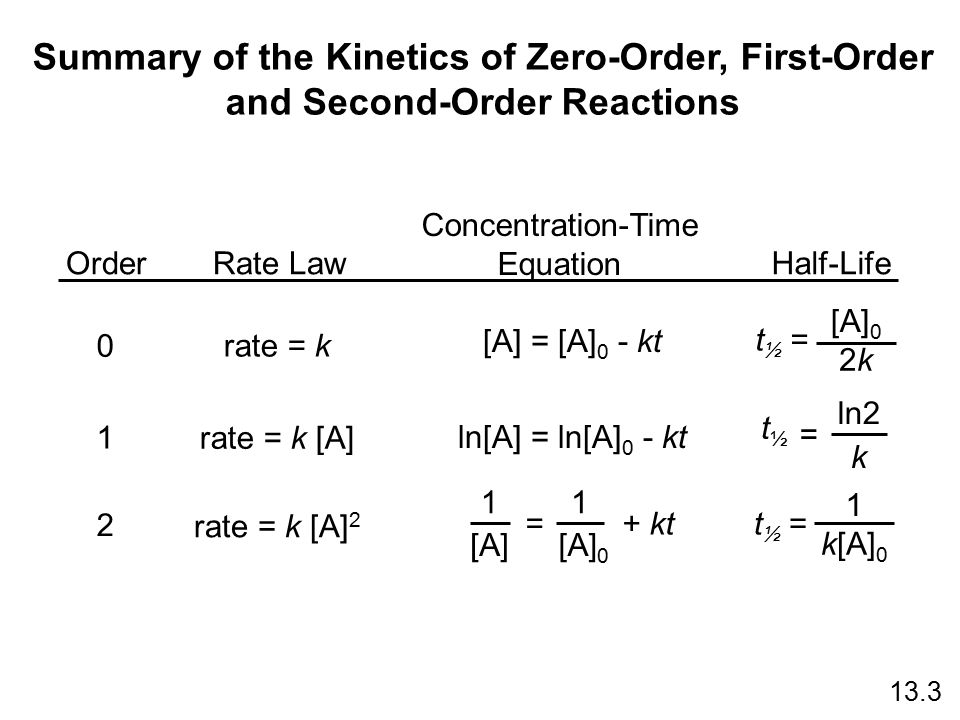
Summary Of The Kinetics Of Zero Order First Order Ppt Download
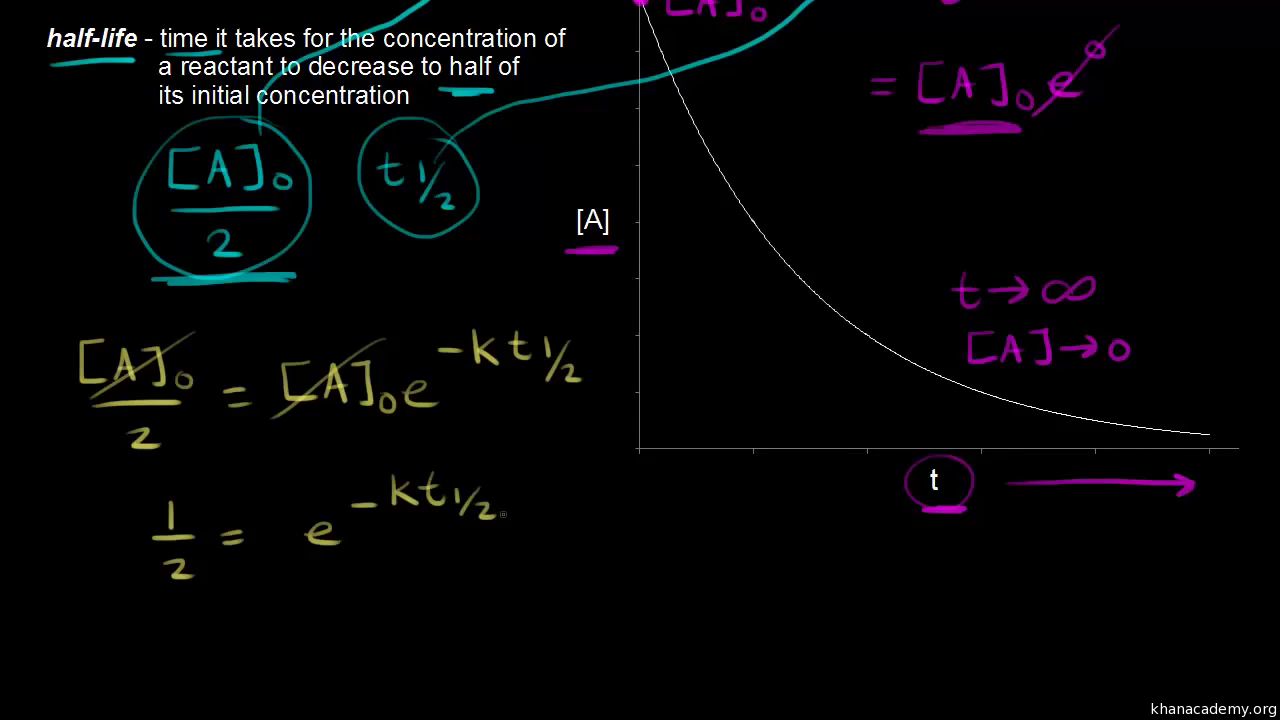
Half Life Of A First Order Reaction Video Khan Academy
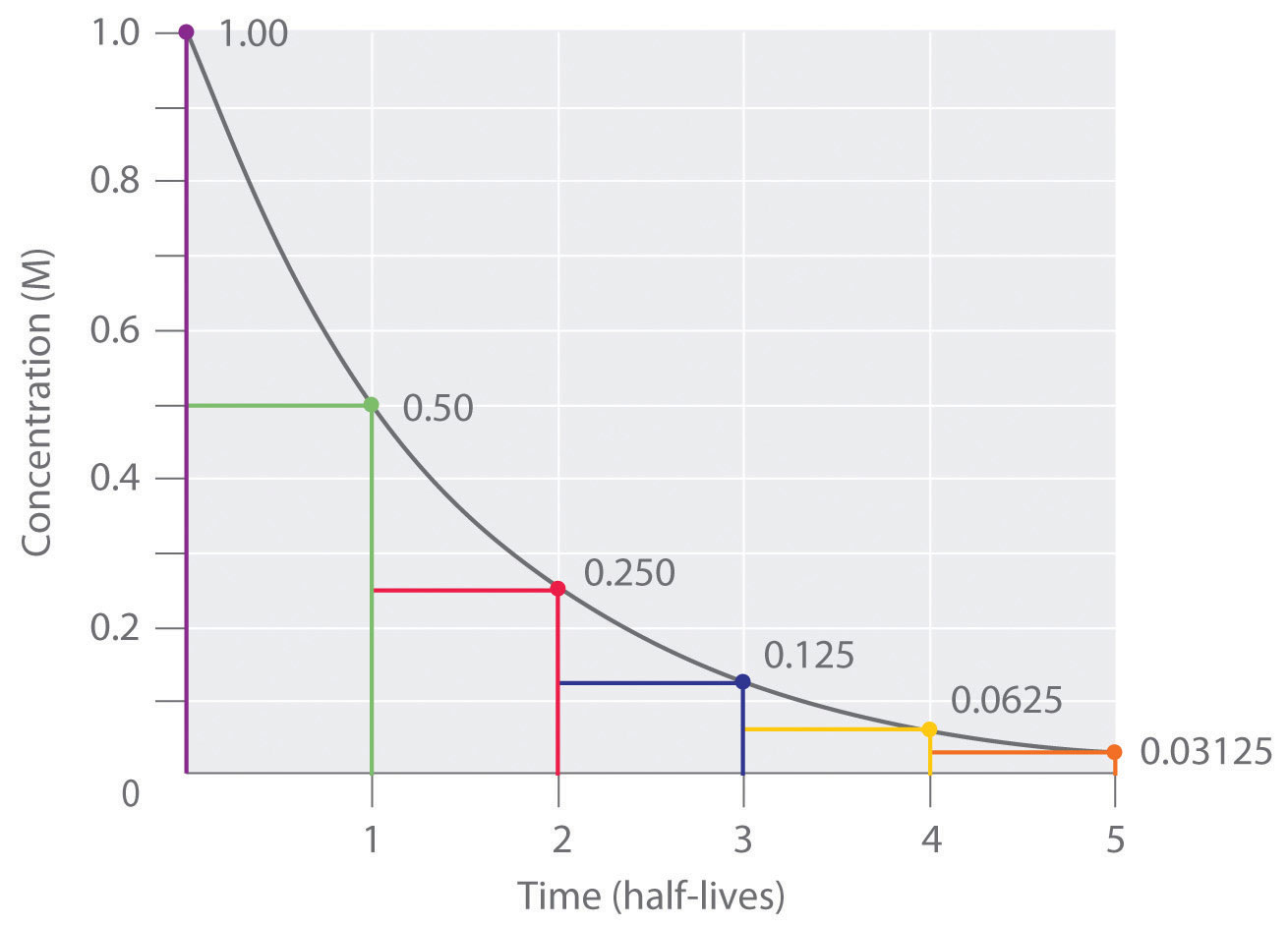
4 5 First Order Reaction Half Life Chemistry Libretexts

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

First Order Reaction Definition Example Half Life Period Chemist Notes

Half Life Of A First Order Reaction Video Khan Academy

Second Order Reaction Definition And Derivation For Rate Law And Half Life

How To Calculate Half Life Of A Second Order Reaction Chemistry Study Com
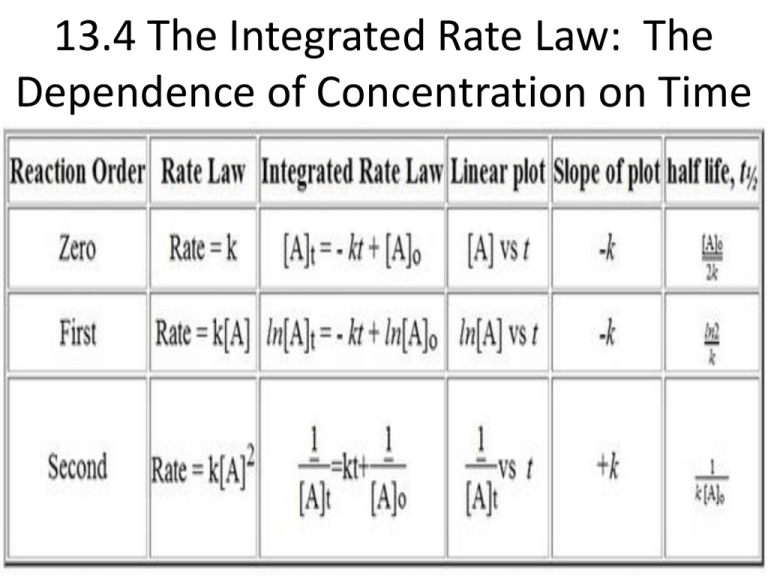
13 4 The Integrated Rate Law The Dependence Of Concentration On

Integrated Rate Laws Chemistry For Majors

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1